How to restore a shorted battery
Over the last century, a huge number of technical products have been invented, the operation of which directly or indirectly depends on the battery.
Some devices require batteries for starting, others for continuous operation. One way or another, the batteries need recharging. Charging batteries is a necessary process in which the charge occurs at a constant value of the charging current; it may differ for different batteries. Any battery has its own service life, declared by the manufacturer, after which the battery must be disposed of.
There are often cases when a battery fails before it reaches its service life, for example, due to a short circuit in one of the batteries. This occurs as a result of the short circuit of the electrical plates, due to the accumulation of sludge at the bottom of the battery.
How to restore a shorted battery?
In most cases, shorted batteries cannot be restored, but those who do not try fail! Kron company specializes in the production of equipment for servicing batteries of all types. To restore closed batteries, the company’s specialists invented charging desulfating devices.
In order to restore a closed battery using a desulfating device, you should never charge it immediately; you must first prepare it.
First of all, you need to rinse the battery with distilled water, rinse until coal chips flow out.
Then fill the battery with electrolyte with the addition of a desulfating additive (depending on the battery volume) and perform the following steps:
- We leave the battery for 48 hours so that the electrolyte squeezes out the air from the cans and the additive has time to dissolve.
- We connect the battery to the charger, but we WILL NOT CHARGE it, we need to drive it through the charge-discharge cycle until the voltage at the terminals reaches the required value. Do not allow the electrolyte to boil or heat up as this may destroy the battery (reduce the charge current if necessary).
- We reduce the value of the charging current by half and continue charging; if within 2 hours the voltage and density of the electrolyte remain unchanged, then we stop charging.
- Add distilled water to the battery to achieve the nominal density of the electrolyte.
- We discharge the battery with a current of 0.5-1 A until the voltage at the terminals drops to 10.2 V (if the battery is 12 V). From the discharge current and discharge time we calculate the battery capacity. If it is below nominal, then repeat the previous points.
- When the nominal capacity value is reached, add another additive to the battery and close the cans.
Our battery is fully reconditioned and ready to use! Contact Kron and we will help you choose the right charger and desulfation device for your batteries!
Reasons for shorting battery sections
No one is immune from troubles, but drivers who do not neglect manufacturers’ recommendations experience them much less often. Moreover, proper operation of the battery does not require any serious waste of resources - you only need to check the electrolyte level several times a year and measure its capacity.
Let's look at the main reasons why the battery shorts out:
- Sometimes there are manufacturing defects, but such cases are covered under warranty;
- often short-circuiting of plates within a section occurs as a result of mechanical impacts (for example, strong impacts) on the battery body;
- The most likely reason for the short circuit of the cans is considered to be a violation of the charging mode - undercharging, if the load on the generator is too high, or overcharging (common with a faulty relay regulator).
All that is required from the car owner to prevent short-circuiting of the battery plates is to keep it clean (this will reduce the amount of current leakage), not leave it inoperative for a long time, and try to prevent the battery from being exposed to severe frost for a long time. Often, because of one inoperative battery, the entire battery has to be scrapped, so the above precautions will not be superfluous.
What to do if the bank in the battery is shorted
Vehicle malfunctions when vehicle operation is prohibited
The question “The battery bank has shorted, what should I do?” This question is most often asked by those motorists whose battery has lasted less than 3 years; the rest simply replace the deteriorated battery with a new one, since restoring “older” batteries is usually a waste of time.
Battery structure (photo).
The feasibility of restoring a car battery.
No matter how loudly the manufacturers of modern batteries shout that today all their products are not disassembled, and therefore not repairable. In some cases, for example, when a good branded battery does not last as long as expected from it, and there is also no money for a new worthy option, it is more expedient for the car owner to at least temporarily restore the existing battery than to buy a cheap Chinese version of unknown quality, especially since moreover, it is quite “real”. After reading this article, you will see this for yourself.
How to identify a shorted battery cell?
Before you take any action on your car's battery, make sure you have the correct diagnosis. To do this, the car battery should be charged. When the battery is fully charged, you will notice the release of air bubbles on the surface of the electrolyte - a boiling process. It won't be in a closed jar.
Also, due to the fact that the short circuit is most often facilitated by the falling of particles from the lead plates to the bottom of the jar, you can try to diagnose this problem visually. Simply unscrew the cap and hold the battery up to a light source. Through the transparent walls of modern batteries, it will be quite easy to see any darkening at the bottom of one of the cans.
When the problem is clearly identified, you can safely begin to eliminate it.
How to restore shorted battery banks - instructions.
Method number 1 is to thoroughly wash the battery and fill it with new electrolyte.
Drain all the electrolyte from the damaged jar.
This must be done very carefully, using personal protective equipment, it’s acid! After draining, make a large cut in the plastic surface (the plastic should be removed strictly from the top of the closed jar). Inside the battery you will see a set of plates that have connections in the form of tracks with neighboring banks
These connections can be sawed (it is convenient to use a hacksaw for this). Remove the plates (immediately mark for yourself how they stood, this is necessary so as not to confuse the polarity at the assembly stage) and rinse them thoroughly in distilled water to remove the acid present inside. Visually determine the location of the short circuit: a lead particle may short between the dielectric and the plates, or perhaps the problem is caused by sediment from the plates, which has accumulated significantly at the bottom of the jar. After completely removing the sediment, lower the plate pack into its original place and connect it using jumpers with adjacent packs - with a soldering iron or a special device. In any case, do not forget to add a lead filler rod to the soldering area. It will make the connection more reliable. Solder the plastic cut off at the initial stage with epoxy resins using adhesive solutions, and then fill the jar with electrolyte and charge the battery. If you did everything correctly, your battery will then start producing 13 V again.
But don’t think that now you can put the battery in a car and drive safely! First, diagnose the entire electrical circuit of the car, because since your battery has failed so quickly, most likely it is being overcharged. If this is not done, any battery you install will have a very short life span.
Method number 2 is to short-circuit the plates of the can.
For all those for whom the first method did not help, you can try the following.
- Cut a hole in the battery cover the entire length and width of the non-working can. Be extremely careful not to touch the walls of “normal” jars.
- Drain the electrolyte.
- Close the 2 contacts between which the plates lie with a metal plate (if you don’t have one, you can use aluminum wire). This is most conveniently done by screwing them onto self-tapping screws.
- Solder the missing part of the lid, pour the electrolyte into the repaired jar and charge the battery.
- As in the previous option, so as not to think about it anymore: the battery bank has shorted, what should I do? – Carry out a thorough diagnosis of the car and only after that return the battery to its place.
Battery malfunctions
This article will focus on lead-acid batteries. You can read about how to restore a gel battery by following the link. Battery malfunctions can be divided into two groups: external and internal.
External
Listed below are external faults and how to resolve them:
- The plastic housing of the battery is damaged. Such damage (crack, hole) can be caused either by external influences or as a result of processes in the battery itself (swelling, overheating, etc.). Here you need to understand that in case of large holes, there is no need to carry out repairs and it is better to buy a new battery. And if the crack is small, then you can repair it using plastic and a soldering iron. Before carrying out work, all electrolyte is drained. When the crack is repaired, you need to fill in new electrolyte and charge the battery;
- The battery terminals have oxidized. Here the restoration task is much simpler. You just need to clean the layer of oxides with fine sandpaper and a rag. It’s a good idea to carry out the same operation on the terminals of the connected wires. After this, you can lubricate the terminals with a small amount of machine oil.
External battery faults
Oxidation of battery terminals
Domestic
Now let's look at the main battery malfunctions caused by internal reasons. Some are quite easy to fix, others are more difficult, and some of the problems are irreparable. First, let's list them:
- Sulfation of battery plates. This is the most common internal battery failure and we will devote most of our recovery recommendations to it. In the early stages of sulfation it can be eliminated without problems, but in the later stages it is much more difficult or impossible. How to revive a car battery during sulfation will be discussed below;
- Shedding of coal plates. This malfunction can be diagnosed by the color of the electrolyte. In this case, it will darken. Here restoration is extremely problematic and you need to prepare to purchase a new battery;
- Short circuit of the plates in one of the battery cans. How to understand that you have this particular malfunction? This can be determined by excessive heating of one of the cans and boiling of the electrolyte in it. The malfunction can be eliminated by replacing the lead plates, but the feasibility of this is questionable. In this case, it would be wise to buy a new battery, since such repairs to a car battery are extremely problematic. Although, below we will consider a method of how to restore a car battery when shorted;
- Electrolyte freezing and damage to battery elements. Improper operation of the device (systematic undercharging, deep discharge) can lead to this result. As a result of leaving a discharged battery in severe frost, the electrolyte can freeze and damage the plates and housing. In this case, restoring the car battery is not practical.
Additionally, you can read about how to check your car battery for performance. As you can see, among internal battery faults, only sulfation is successfully eliminated. Moreover, if it is not in an advanced stage. Therefore, we will look at several ways to restore a battery in case of sulfation of lead plates. But first, let's list what we may need for work:
- Fresh electrolyte;
- Distilled water;
- Battery charger;
- Hydrometer (measurement of electrolyte density);
- Protective equipment (glasses, gloves);
- Desulfating agent and some other chemicals.
Battery leakage detection
Causes and Symptoms of faulty spark plugs and ignition coils. Signs of faulty ignition coils
- Remove the “-” terminal from the battery
- We connect one of the ammeter probes to the “-” battery
- Another on the removed wire (polarity on the digital multimeter does not matter)
- Disconnect the positive terminal from the battery
- Connect the ammeter with the negative terminal - to the contact terminal of the car
- Positive terminal - to the battery
- Prepare the car for testing (turn off the radio, side lights, interior lighting, etc.)
- After a minute, connect the ammeter to the open circuit and take readings. (the peculiarity of car alarms is that they become armed no earlier than after a minute)
- As we saw the leakage current on the ammeter, we begin to pull out and put back the fuses and relays in order.
- It will become clear which circuit is leaking when the current returns to normal.
Accelerated Recovery Method
If you are accustomed to value your time, then take a closer look at the method of accelerated battery resuscitation, which is as follows:
- Charge the device to full.
- Drain the filler solution.
- Clean the inside of the appliance using distilled water.
- Pour in a two percent solution of Trilon B and a five percent solution of ammonia.
- After 60 minutes, drain the mixture and rinse the battery thoroughly.
- Pour in fresh acid solution.
- Place the device on charge.
Remember that measures to prevent battery breakdowns and malfunctions are much more effective than measures for subsequent repairs.
Try to keep the terminals and leads clean and charge the device to full once every six months. Such prevention will extend the service life of the device to 7 years.
How to check a car battery
Car generator malfunctions
Even in a specialized store, a new recharged battery must be checked upon purchase. First, carry out a visual inspection for damage to the housing and the condition of the terminals.
When buying a battery in a store, you still need to check it
The abundance of counterfeits forces you to check the date of manufacture, the country of manufacture and the name of the company, including the address, when purchasing even an expensive battery. Batteries that were filled no more than 3 months ago are considered “fresh”. When not used, the batteries spontaneously discharge. which will negatively affect the overall battery life.
Secondly, it is advisable to ask the sales consultant to measure the density of the electrolyte - the correct percentage of sulfuric acid and distilled water. A serviceable and fully charged battery should have an electrolyte density of 1.24 g/cm3 in summer and 1.28 g/cm3 in winter. A decrease in the declared density by 0.02 g/cm3 indicates that the battery is 80% charged.
Checking a serviceable and charged battery with a voltmeter with the load off should show 12.5 - 12.9V, and with the load on, it should not fall below 11V for 10 seconds.
Just add water: how to revive a dead battery
Serviced or not?
The electrolyte of lead batteries consists of two components - sulfuric acid and water.
Water, which evaporates over time, is to blame for the decrease in electrolyte levels. As a result, some of the plates are not immersed in the electrolyte, and the battery loses capacity. If in summer this effect can be ignored painlessly, in winter it will certainly give you a frosty morning pig... It is customary for car owners to divide batteries into “maintenance” and “non-maintenance” according to the type of caps on banks. If the plugs are present and can be unscrewed with a coin, it means it is “serviceable”: you need to monitor the electrolyte level and add water if necessary. If there are no traffic jams, it’s the other way around.
Articles / Practice Light a cigarette - throw away the battery: myths and truth about batteries in winter Winter has arrived, and with it traditional battery problems. Both advanced car enthusiasts and lazy drivers encounter them: cars with open hoods are waiting to “light up”... 109971 10 12 12/22/2016
- In fact, “maintenance-free” lies primarily in the fact that the battery is made with calcium additives in the lead electrodes instead of the good old antimony, which has been used for decades, says Alexander Kazunin, head of the battery laboratory of the Research Institute of Automotive Electronics and Electrical Equipment.
- “Calcium” batteries have a very low rate of electrolysis of water, which almost does not evaporate from the electrolyte under normal operating conditions. And therefore, they often lack plugs to control the electrolyte level. However, you need to understand that with the advent of “calcium” batteries, the problem of electrolyte boiling away did not completely disappear. “Antimy” batteries, which are prone to a drop in the electrolyte level, are still produced and sold, and “calcium” batteries can easily require monitoring and topping up if the car is driven intensively in the summer in the city cycle or, say, the voltage regulator in the generator is faulty.
Calcium can be applied only to the negative electrodes of the battery or to all electrodes. Batteries in which all electrodes are doped with calcium are called “calcium-calcium” (Ca/Ca). True, the price to pay for the lack of maintenance of the electrolyte level is increased sensitivity to deep discharge. A “calcium” battery, once set to “zero”, as a rule, does not last long...
About water
Often, even in truly maintenance-free batteries, there are still plugs, but they are not separate, but attached to a common plastic plate, which is covered with a branded sticker on top. There are no obvious signs on these plugs that they can be opened. But this can be done, and often necessary. Because the electrolyte level can drop in almost any type of battery.
Leveling a low electrolyte level in a battery is easy and inexpensive. It is enough to purchase a bottle of distilled water from a car store and add it using a syringe or bulb to each battery jar, the number of which is six for a car with a 12-volt electrical system. Looking into the jars with a flashlight, you can see the plas, which is a level mark. If it is not there, water is added until the plates are completely covered. After this, it is highly advisable not to load the battery with the starter, but to recharge it.
Articles / Practice We charge a dead battery in 10 minutes: experiment Kolesa.ru Many of those who are not shy about getting into the “guts” of their car with tools know in practice about a very curious, useful, but at the same time strange and poorly explained... 237111 9 62 04/18/2016
This procedure is simple and accessible to any car owner. The only bottleneck in this story is the purchase of distilled water. Typically, the “distillation” packaged in 1.5-liter bottles is produced by companies like “Horns and Hooves,” and it is not so easy to find water produced by a well-known brand of automotive chemicals on sale. And in view of the low retail and even lower purchase price of distilled water, manufacturers have a serious temptation to reduce costs as much as possible and start dispensing tap water under the guise of distillation for batteries... Moreover, a deceived buyer is unlikely to make a claim: the battery is from ordinary water, will certainly die, but this will not happen instantly.
Here is a typical review of low-quality distilled water from one of the UAZBUKI forum members:
“Once I had an unopened bottle of this water lying in my trunk. She probably lay there for four months. And somehow I decided to add it to the cooling system. I opened the bottle and it smelled like rotten stuff - at least run away. What swamp did they get it from..."
TDS meter
You can check the quality of purchased distilled water using different methods. The most correct way of checking available at home is to use a specialized device called a TDS meter. Chinese online stores are full of them, they are not too expensive, and the accuracy is quite sufficient for our needs. The TDS meter looks like a pencil with a display and measures the level of total mineralization (salt content) of water in “ppm” units - the number of particles of dissolved salts per million particles of an aqueous solution.
We measure tap water - 215 ppm. We measure distilled water from a car store - a bottle from one manufacturer shows 8 ppm, a second – 7 ppm, and a third, the one that says “double purification”, shows 0 ppm!
Respect to the last manufacturer, of course! The product is really high quality. But even if the ppm of distillation is not zero, there is no need to worry. A small number is within acceptable limits. In the end, almost any Soviet textbook on automotive operating materials, as a last resort, allowed the use of melted snow water for the electrolyte (not from city snowdrifts, of course), the ppm of which is usually 10-20.
1 / 4
2 / 4
3 / 4
4 / 4
Ohmmeter
Many sources suggest checking the quality of distilled water with a multimeter in ohmmeter mode. In other words, simply by measuring its resistance. Often there are even numbers: if the water resistance is more than 30 kilo-ohms, this means the water is suitable for the battery.
At first glance, it looks reasonable: a multimeter, unlike a TDS meter, is found at home or in the garage much more often than the latter. And the TDS meter calculates the number of ppm indirectly, precisely through measuring the water resistance.
But there is a fundamental difference: a TDS meter measures resistance on alternating current, and an ohmmeter measures resistance on direct current. And electrochemical processes that begin in water when direct current is passed introduce very large errors. And when we add to them the completely random geometric dimensions of the ohmmeter’s measuring electrodes, and the distance between them, taken by eye, the parameters begin to jump chaotically, changing tens of times. So you shouldn’t use a multimeter to assess the quality of the distillate.
Evaporation
The next method is visual. It is unlikely to give a clear assessment of the quality of the “distillation”, but at least it will allow you to identify outright fraud when, under the guise of demineralized water, they slip you tap water.
For this test we need a clean piece of glass. We drop two drops of water on it next to each other: what we consider to be distilled, and tap water for clarity. Then we wait for the water to evaporate, which can be accelerated by heating the glass on the lighter. After evaporation, distilled water leaves virtually no salt stains, the stain simply disappears. If obvious salt “circles” are noticeable, the water is most likely from the tap...
In the photo on the left there is a salt stain from tap water, on the right nothing is visible - a drop of distilled water has evaporated there.
220 volt
And finally, one more way. Severe Chelyabinsk - checking water resistance on an alternating current electrical network of 220 volts. As it becomes clear, it is based on the fact that ordinary water conducts electric current, while distilled water practically does not. This is also a conditional test that does not give results in digital form, but is quite suitable for everyday use, and most importantly, it is visual. The procedure is quite simple, but requires some caution when handling exposed live wires!
We assemble a simple circuit from an electrical cord with a plug and a socket for a 220-volt incandescent lamp. Approximately in the middle of the double cord, cut one of the wires and strip the ends. Now the cut ends serve only as a breaker. We screw in the lamp, insert the plug into the socket for testing - the lamp burns at full intensity. Now we take out the plug, cut one of the wires of the pair, strip both ends to a length of about a centimeter each and lower these ends into a glass of test water. Re-insert the plug into the socket. The lamp will not burn on distilled water, but on tap water its filament will glow dimly, dimly, at less than a quarter of the incandescence.
Well, now that it is clear which water is truly distilled and which is not, the only thing left to do is add the “correct” water to the battery. And in the same way as we described above. And enjoy the good battery performance.
Survey
What do you do if the battery does not spin well?
Your voice
Total votes:
How to identify a closed jar
There are several ways. Let's just say there are technical ones, and there are philistine ones.
Technically, you just need to take and measure the voltage on each of the banks with a “multimeter”. However, this was easy to do with old Soviet batteries; there, the jumpers between the banks were on top, and you could easily determine the closed version. But modern batteries are completely filled with plastic, it is almost impossible to get to the jumpers!
The second option is to measure the voltage on the entire battery, if in the charged position it is no more than 10 - 11V - you know, it has shorted the bank, it makes almost no difference which one.
But how to determine the full charge in other banks?
A simple method is that a charged jar, when fully charged, begins to emit gas (bubbles appear). If five pieces “bubble” and one doesn’t, then that’s the problem.
The common way is to simply look at the color of the electrolyte inside. Unscrew the plugs, if any, and look inside. In a “closed” mode it will be black (or dark) in color, which indicates its inoperability. Of course, modern batteries are not collapsible and they don’t have lids (although if you poke around, you can find them), but they are usually transparent, made of white plastic, and if you look at the sun, you can see the cloudy part, thus identifying the jar that is shorted.
Battery care tips
In order for the battery to work without interruption, you need to constantly and competently care for it and follow simple tips:
- You will have to regularly monitor the electrolyte and its density. The main reason for boiling is overcharging or overheating of the device. The sooner the problem is detected, the greater the chance of recovery.
- If the car is not used in winter, the battery must be removed and placed in a well-heated room. If the battery freezes, it will no longer be possible to restore it.
- The rated current is 0.1 of the capacity of the entire battery. If this threshold increases, the device will not be restored.
We recommend: Characteristics of the Bosch s5 car battery
These are very simple tips for maintaining your battery in good condition. They should not be neglected. Only in this case will the battery work for many years without interruption. Restoring a car battery is not a difficult task, but it will take some effort and studying the main faults. After studying all the tips, you can understand how to restore a car battery on your own.
So what to do now, is it possible to restore it yourself?
For a simple driver (especially if he is a beginner), it will be difficult to restore, I would even say impossible - it’s better to hand over and buy a “new” one.
But for the “Kulibins” whose hands are itching, there are small instructions for restoration. Just be careful, there is acid inside!
SO, what we need to do:
1) We determine a non-working, closed jar (total voltage is about 10 - 11 Volts, there are no bubbles when charging, it is dark inside).
2) We drain the electrolyte from it.
3) Cut the plastic around it, always from the top.
4) Disconnect the jumpers with adjacent banks, you can also cut them.
5) We take out the package of plates and rinse it with water, this is necessary to wash away traces of acid.
6) Now we need to determine the location of the closure:
First, we carefully examine each plate and the dielectric between them. We need to find a particle that bridges them.
Secondly, if you haven’t found one, then perhaps it’s all about the sediment that has accumulated at the bottom, just rinse it - clean out all the lead “dirty” at the bottom.
7) After we are convinced that the plates are in order, the dregs have been removed, we lower the “package” back into the jar.
8) Solder the jumpers to the adjacent packages, fill in the electrolyte, close the plastic cover that we cut off. It needs to be melted hermetically, possibly using adhesives and epoxy resins.
9) Now we need to charge our battery. In 90% of cases, the voltage rises to the desired level, about 12 - 13V. That is, it can again be used on the car.
This is how several of my friends restored them, and you know, they worked for a very long time!
Therefore, a closed bank is not always the death of your battery, sometimes it is possible to revive it, the main thing is to have the right hands.
That's all, read our AUTOBLOG.
(10 votes, average: 3.70 out of 5)
Similar news
What is battery capacity. Car or phone, as in.
How to charge a gel battery. Charger and from the car.
How much does the battery weigh? Let's look at car options from 55.
The battery is the most significant part of the car (if its soul can be expressed that way), without a battery the driver simply will not be able to start the current power unit, if the old batteries could easily be started with a “crooked starter” (a special key), then the new ones are hung with electronics, which one will not allow the start.
In general, a rechargeable battery is extremely necessary, but unfortunately, it does not last forever and can break down for various reasons. One of the most common problems is the bank shorting out. After such a problem, the power source is thrown away or sold for scrap in 90% of cases.
Recovery due to incorrect usage
No matter how strange it may be, you also need to restore the battery in your phone due to improper use of the device.
In this case, we mean an interrupted charging and discharging cycle, use in high or low temperature conditions, charging using “non-native” devices.
Such methods of use cause serious harm to the power source, and its internal resource is used up faster.
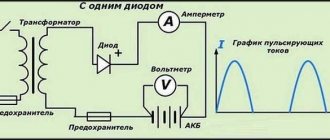
Measuring voltage with a multimeter
If you do not want to check the condition of the battery “by eye”, or simply want to have accurate results in numerical terms, you will need a device such as a multimeter.
So, how to check a car battery with a multimeter: the device itself has two probes, one of which is red and the other black. In order to measure the voltage on the battery, you need to put the multimeter in measurement mode and place the red probe against the “positive” terminal, and the black probe, respectively, against the “negative” terminal.
The procedure is carried out on the battery terminals without load. If the battery is properly charged, the result on the device will be about 12.6-12.9 volts. This is the normal voltage that should be present on a fully charged battery. If you mess up the colors of the probes, the number will be the same, it will just be displayed with a minus sign.
If the engine is running, then you can also check the operation of the battery with a multimeter, but in this case it will be checked whether it is working together with the generator, as well as the serviceability of the voltage regulator. When the motor is running, the readings should be slightly higher - from 13 to 14 volts. If the indicator is lower, this will mean charging problems for the battery, and if the indicator is higher, the process of water electrolysis will begin.
There is a method to determine the degree of battery discharge by voltage. Voltage 12.5 - says that the battery is 90% charged, voltage 12.1 - 50%, and 12 - 10 percent. Although the method is approximate, it is proven.
It is also advisable to measure with the device “cold”, since a car that has recently been running can give higher values and be misleading. The multimeter checks the degree of charge, but does not provide comprehensive information about its performance. To do this, it is better to use a load fork.
Low current restoration
How to bring your car battery back to life and revive it? This device provides continuous current transmission to power the electrical equipment of the vehicle. Accordingly, without this device, normal operation of the devices will be impossible, especially since over time the battery can no longer hold the nominal charge required for power. Not all batteries that work poorly need to be thrown away - you can try to revive an old battery. This will avoid unexpected financial costs.
Design and designation of battery design components
If we talk about acid-base batteries, the structure is several positive and negative lead plates in sulfuric acid. Today, devices of this type are the most common among cars used in the countries of the former USSR. Despite its prevalence, batteries have a shorter service life.
Restoring a car battery with your own hands can be done using repeated charging technology. In this case, a small current should be used. The charging procedure using a charger-restoring device must be carried out intermittently. From the first charging of the device to the last, the voltage level present in the battery will gradually increase. As a result, the device should stop discharging.
The charging recovery device should work with pauses, this will allow the potentials of the electrodes located in the plates to equalize. The restoration procedure itself is safe for electrodes. Using a charge-recovery device with pauses will ensure the transition of the densest electrolyte from the plates into the space between the electrodes.
Unscrewing battery can caps
As a result of the use of partial discharge technology, an increase in battery capacity helps to increase the density of the electrolyte. The car owner is required to wait until the moment at which the voltage corresponds to 2.5 volts and the density parameter corresponds to the nominal value. And in this case, we must not forget that the car battery needs a break, so the charger and recovery device must be periodically turned off. For complete resuscitation, the cyclic recovery procedure must be repeated 8 times. It must be taken into account that the current used should be 10 times less than the capacity of the charged battery.
Why the battery fails Main reasons
Explosion of a car battery as a result of its overcharging
Overcharge and undercharge
The first reason mainly occurs when driving short distances. The battery simply does not have time to charge to its nominal value. If there is a fault in the wiring or electrical equipment, charge leaks, which weakens the battery. Do not forget about turning on the radio or low beam lamp. This also drains the battery. Read more in the article: Reasons for the rapid discharge of a car battery.
Low electrolyte density
The second reason for poor performance of a car battery is the low density of the electrolyte. You can check it with a special aerometer device. But this check can only be performed on serviceable batteries where there is access to the battery banks. There are fewer of these batteries being produced. Mostly maintenance-free batteries are on sale.
Electrolyte Density Table
Recovery due to malware
In a certain sense, the scourge of all smartphones is running the Android OS, since this platform is very popular with people who are not very scrupulous.
It’s quite easy to catch a virus – all you need is Internet access and a few careless clicks on dubious links.
Viruses can not only steal your personal data (including financial data), but also run many programs in the background.
Usually they may not even be displayed to the user, but they work and consume battery resources. This leads to the fact that after some time it will be necessary to carry out the battery restoration procedure.
Where to check the battery status
The best option for checking the condition of a car battery is professional testing of all parameters in a car repair shop. The presence of all the necessary equipment will allow us to make an unambiguous conclusion about the possibility of resuscitating the battery.
The working life of any battery is not unlimited and depends on operating conditions. An average annual temperature of +4°C with a mileage of 30 thousand km/year will allow the battery to last a little more than 2 years. Annual mileage of up to 20 thousand km/year extends the battery life to 3 years.
To check the battery operation, it is better to contact a car repair shop.
Predominant driving on broken roads, constant shaking and vibration make it advisable to purchase a maintenance-free battery to avoid short circuits. For trouble-free starting of powerful Japanese SUVs with installed subwoofer, air conditioning, heated steering wheel, seats and windows, you will need a battery 5-10 Ah more powerful than recommended by the manufacturer.
What is battery calibration and how can it help with recovery?
Of course, it's much better not to let your power supply get to the point where it needs to be restored.
It is much easier to use preventative methods - such as battery calibration. It will be useful for both new batteries and those that have already begun to lose their former properties.
Most modern batteries recommend not allowing them to fully discharge, and also not charging them to 100%. Because of this, the battery does not know how to behave correctly. The essence of calibration is precisely to show the battery how to work.
In this case, this technique is used. First, the phone is discharged to 0% so that it turns off on its own, and then it is put back on charge and not removed until it is 100% charged.
Even after this time, you need to wait several hours to ensure that the battery receives all the necessary power. Only after this procedure can it be turned on.
This example that we described can be called “mechanical battery calibration”. There is also a programmatic way to do this, because for most people this will be preferable to waiting half a day.
To do this, you need to go to Google Play or the App Store (this depends on what OS your phone is running - Android or MacOS) and enter in the program search: “battery calibration”.
We do not provide any specific examples, since by the time you read the article they may either be outdated or not suitable for your phone at all.
And by entering such a search query, you will always receive only the latest and current version of the battery calibration program for your smartphone.
Checking the battery with a load fork
The load fork is a very precise tool, but is rarely used in “manual” use, as it is used mainly in service stations. The beauty of this method of verification is that thanks to it an absolutely accurate result will be known.
The load plug works in a similar way to a multimeter: it is also connected to the battery terminals, but produces a short circuit current. As we already said, without load the indicator should approach 13 volts. The load fork imitates the operation of a starter, which is why the voltage “sags” at the moment of use.
The drop should be no more than 9 volts. Otherwise, it will mean that the battery is very discharged. After removing the load, the indicator again “rolls back” to its original value. If under load the voltage “sags” to 5 or even 3 volts, this means that the battery will not be able to start the car engine.
It is recommended to think about changing the battery even if the voltage when using the load plug drops below 9 volts.
An extremely important note: the load plug supplies approximately 200 amps of voltage to the battery and is not recommended for use in low temperatures. Ideal conditions for “field” use of this device is a temperature of +20-25 degrees Celsius
If you apply voltage to a cold battery, there is a risk of severely discharging it.
Battery Maintenance
As you can see, there are several answers to the question “how to reanimate a battery.” But in conclusion of the article, we would like to give not just another piece of advice on how to revive an old battery with your own hands, but recommendations on how to maintain your battery. By following these simple rules, you will significantly extend the life of your battery, and, most likely, you will not have to resort to the advice from our article.
So, what does battery maintenance include:
- Periodically clean the battery from dirt, electrolyte residues and oil.
- Secure the battery carefully.
- Check the cleanliness of the gas outlets and clean them if necessary.
- Clean terminals and wire ends.
- Monitor the electrolyte level in the battery and periodically check its density.
There is nothing complicated about battery maintenance. But these simple rules will keep the “electric heart” of your car healthy for a long time, and we hope you will never need battery resuscitation!
Reasons for rejecting acid batteries
An acid battery is a dynamic system with a continuously ongoing electrochemical reaction. It is this that creates the conditions for receiving energy for storage and transferring it to the consumer. But as a result of a continuous process, the internal components wear out and are continuously transformed. Parallel to the useful ones are parasitic reactions that accelerate the process of device degradation.
As a result of violation of the operating instructions for the device and for objective reasons, the functionality of the battery is impaired, and the following occurs:
- sulfation – deposition of a crystalline deposit of lead sulfate on the plates, preventing the accumulation of charge;
- destruction of the lead plate, coal grate and shedding of the active mass to the bottom;
- short circuit inside the can and between the body and the plates, caused by mechanical damage or internal short circuit by sludge;
- destruction of the battery case by a sharp blow, explosion or freezing of the electrolyte.
Regardless of the reason that caused the signs of rejection, the product loses its ability to produce current of the required parameters. It is possible to restore an acidic battery by desulfation - the destruction of poorly soluble sediment by chemical and physical methods. Let's consider several methods of electrical action that destroy sediment and restore the functions of an acid battery.
What to look for
It will be useful for motorists to understand how to find out whether they really have a shorted battery or not.
A classic battery uses lead plates and an electrolyte made from a mixture of distilled water and sulfuric acid. The battery consists of 6 sections. They are called banks. Each jar contains a pair of plates with different polarities. The contacts are collected into a common collector and connected in series. Two terminals are located on the housing cover.
Modern batteries may have a slightly different design. But the layout and equipment of the cans, as well as the cells, remains unchanged.
When the sections are connected in series, the voltage from each bank is summed. Capacity parameters are determined based on the most clogged cans.
To charge the battery, voltage must be applied to the terminals. As a result, the current begins to spread across individual sections.
The question is where the short circuit comes from. There are several options here. The oppositely polar plates come closer and contact each other. The walls swell, a jumper is formed, which leads to a short circuit. A contact bridge can also appear as a result of grease shedding.
You also need to understand that a car battery is truly closed. To do this, check the voltage at the terminals by connecting a voltmeter or a universal multimeter to them. When measuring the voltage, a dip is detected.
As you know, a total of 6 cans of 2.1 V each give 12.6 V. In theory, the battery is fully charged. But the test shows only 10.5-11 V voltage. If this is the case, then you need to look for the problematic section.
The plates inside have a lead base, but are additionally coated with special active substances. If the electrolyte level drops, the battery is overcharged or deeply seated, then the mass often crumbles, which leads to a short circuit.
The possibility of external mechanical damage cannot be excluded. The motorist accidentally dropped the battery while removing or installing it. Or there was an accident. Hence the violation of the internal structure with the resulting short circuit of one or several cans at once.
There were also cases when the electrolyte inside froze. When frozen, the liquid expanded and deformed the walls, damaging the plates. They touched, which became the reason for the short circuit.
Checking the battery voltage
There are several simple signs of how to determine a short circuit in a car battery. This is a check of the voltage that has dropped, as well as the integrity of the case and its temperature. If the battery gets very hot and the walls are swollen, there are few options.
When the battery does not want to be charged even to 12 V, and does not crank the starter, then you will have to change it.
In rare cases, you may notice the smell of rotten eggs when charging. This indicates that the release of hydrogen sulfide has begun. An extremely dangerous phenomenon. Charging must be stopped immediately and the battery itself must be disposed of.