The operation of the battery in storing and consuming energy is based on a reversible electrochemical reaction. In this case, balance must be maintained, all components must participate in energy exchange. Sulfation is the formation of an insoluble deposit on the surface of the battery plates in the form of a solid coating. Lead and the acid residue SO4 are removed from the process, and the electrolyte concentration is reduced. When deposited on the plates, the sediment increases resistance and interferes with charge transfer. As a result, the device loses capacity. How to detect and eliminate battery sulfation?
How to determine battery sulfation
The reasons for the appearance of white deposits on the battery plates, sulfation, are associated with violation of proper operation. During the discharge period, PbSO4 crystals are always formed, but they are small in size. When charging the battery, they are ionized again, and the conductive surface is cleaned.
Sulfation of battery plates occurs if there are reasons:
- A deep discharge leads to the enlargement of crystals, which are not destroyed during charging.
- Low temperatures lead to chronic undercharging of the battery. A cold electrolyte slows down the rate of chemical reaction. If trips are short, downtime is long - all the prerequisites for battery sulfation.
- High temperatures in the engine compartment in summer accelerate all processes, including the formation of large crystals of lead sulfate in a discharged battery.
- Storing an undercharged acid battery will cause the crystals to gradually grow and thicken as a result of self-discharge. In this case, recharging is not performed, the crystals are not destroyed.
- Low level of electrolyte in banks, poor quality of electrolyte.
- Adding concentrated acid to reduce sulfation will only increase the size of the clogged surface.
The sooner you determine the appearance of sulfation on the plates of an acid battery, the easier it is to destroy the sediment and free access to the receiver of charged particles. How to do it?
It is necessary to periodically inspect the cans of a maintenance-free battery - a brownish-whitish coating on the plates is clearly visible through the open cap. Sulfation leads to loss of capacity. Clear signs: the car battery is charging within an hour, the cans are boiling. After charging the battery does not start the engine, it is quickly discharged by the backlight. A white coating forms on the body, around the plugs, and on the terminals; the electrolyte boils in the battery installed in the socket. The battery capacity decreases; this can be determined by measuring the voltage at the xx terminals and under load.
All of the listed signs of sulfation are also characteristic of maintenance-free calcium batteries, but to a greater extent. Two or three deep discharges, and the calcium battery will become completely unusable. Not only lead sediment is formed here, but gypsum, which is worse. The problem manifests itself as a decrease in capacity and short charging time.
Video
We invite you to watch a useful video about battery sulfation.
The positive plates become light brown in color and white spots appear on the surface of the plate. The negative plates become whitish-gray and swell. Battery sulfation causes a significant increase in the resistance of the active mass of the plates and, consequently, the overall resistance of the battery. As a result, the voltage of a sulfated battery at the beginning of charging may increase at normal charging current. In this case, the battery immediately begins to “boil”.
With very deep sulfation, when the sulfate forms a continuous crust on the surface of the plates, the battery may completely lose conductivity.
Possible causes of sulfation:
1 Temperature fluctuation.
Lead-acid batteries are not as harmfully affected by low or high temperatures as by temperature fluctuations.
The fact is that lead sulfate has relatively low solubility in a solution of sulfuric acid. The intensity of this solubility increases greatly with increasing temperature. As the temperature increases, part of the sulfate located on the plates dissolves, and upon subsequent cooling, the sulfate begins to fall out of solution and settle on the plates. The sulfate falling out of the solution will mainly settle on those places on the surface of the plates where there are already small particles of crystalline sulfate. With repeated fluctuations in the temperature of the solution, this process will also be repeated, as a result of which small particles of sulfate will gradually turn into large crystals of lead sulfate (sulfate), which are not reduced with a normal charge. In this case, the battery capacity is reduced not only due to a decrease in the amount of active mass that took part during discharge and charging, but now, having turned into coarse crystalline sulfate, has become inert. The capacity of the battery also decreases because the active mass lying under large crystals cannot take an active part in the reactions accompanying the processes of charge and discharge.
The process of sulfate formation under the influence of fluctuations in solution temperature is characterized by the fact that large sulfate crystals form slowly and not so much on the surface of the plates as in the depths of the pores of the active mass. This is especially detrimental to the positive plates, the wear of which is therefore accelerated.
Read also: At what height should you hang sconces above the sofa?
2 Increased density of sulfuric acid solution.
Meanwhile, in some cases, maintenance personnel, when detecting batteries with a reduced density of sulfuric acid solution, without thinking about the reasons for such a decrease, add sulfuric acid solution to the battery instead of water. This cannot be done, since a decrease in the specific gravity of the solution in some batteries is most often associated with the phenomenon of partial sulfation and does not require adding sulfuric acid solution, but treating the battery - eliminating the sulfation that has begun.
During the next equalizing or control discharge of such a battery, the density of the sulfuric acid solution is usually significantly higher than that of other batteries.
The presence of an excessively large amount of sulfate prevents the normal diffusion of the sulfuric acid solution, uneven distribution of sulfate occurs in the layer of the active mass of the plates and its normal recovery during subsequent charging is not achieved.
3 Reduced level of sulfuric acid solution in batteries.
The sulfuric acid solution should always cover the plates so that the top of the plates is not exposed. If, due to an oversight or a leak in the battery vessel, the level of the sulfuric acid solution drops for a long time so much that the upper part of the plates ends up in the open air, then this part of the plate after some time may become unusable due to deep self-discharge (sulfation), and then under the influence of the remaining in the pores sulfuric acid may simply undergo destruction.
If part of the plate was exposed to air for a short time, then after adding water to the normal level, such plates can be returned to normal. When exposed to air for a long time, the negative plates are especially affected, the active mass of which turns into a liquid pulp, creeping out through the perforation and falling to the bottom of the vessel.
Leaving batteries in a discharged or undercharged state for a long time. Charging of discharged batteries must begin no later than 12 and, in extreme cases, 24 hours after the end of the discharge.
But if 50% discharged or, even more so, completely discharged batteries are left for a long time without a charge, then battery sulfation occurs. The process of sulfation of sponge lead in negative plates is especially intense. Here, many tiny particles of this lead (unused during the discharge) are gradually sulfated due to the large surface of contact with the acid. In this case, an outwardly unnoticeable release of hydrogen occurs. The higher the temperature and density of the sulfuric acid solution, the more intense this process occurs. Sulfate crystals grow deep in the pores of the active mass of the plates. From fine-grained it turns more and more into coarse-crystalline, almost impossible to restore with subsequent normal charges.
Prolonged stay of batteries, estimated in months, not only in a discharged state, but also in an undercharged state, leads to a loss of capacity due to the appearance of coarse-crystalline sulfate in the active mass of the plates of both polarities.
It would be appropriate to remember that after any discharge, deep or not deep, short-term, but with a high current, or long-term with a low current - under all conditions, the battery must be charged in order to obtain a complete recovery and should not be subjected to significant discharges again.
The results of keeping batteries in a discharged state for a long time are similar to the state when the batteries are systematically undercharged. In an undercharged battery, some amount of lead sulfate remains in the active mass. If undercharges are repeated with each charge, then the sulfate crystals will continuously grow in size and quantity. It is easy to deal with undercharging - you must follow the most important rule for batteries: the charge of the batteries must be full and must be checked according to all signs - by the amount of capacity received during discharge and returned during charging (taking into account the return coefficient), by long-term constancy of voltage and density of the electrolyte solution in at the end of the charge, also by uniform gassing (“boiling”) of the plates of both polarities.
4 Frequent deep discharges.
When operating batteries in the charge-discharge mode, the best results for protecting batteries from sulfation are obtained if the capacity is systematically removed from them does not exceed 75 - 80% of the nominal.
5 Frequent charges with large currents.
When charging batteries with a large current, not all the sulfate on the plates has time to decompose, because electrochemical reactions are different in that they require a certain (and quite long compared to conventional chemical reactions) time for their implementation. With a high charging current, signs of the end of the charge (constancy of voltage and specific gravity of the sulfuric acid solution) may occur before complete reduction of sulfate on the plates of both polarities is completed.
In the pores of the active mass of the plates, at high charging currents, the density of the sulfuric acid solution increases strongly and intensively. In a concentrated solution, sulfate, without decomposing, easily dissolves, and during a subsequent discharge, due to a decrease in its concentration in the pores, the sulfate falls out of the solution in the form of crystals, which, with a normal charge, do not turn into spongy lead and lead dioxide. Thus, the amount of active mass decreases, and so does the battery capacity. The intensity of these processes harmful to the battery is also facilitated by the fact that when charging with high currents, the temperature of the battery increases significantly more than when discharging with currents of the same magnitude.
Read also: Cutting splines on a machine shaft
Methods for eliminating sulfation.
In order to prevent the formation of large amounts of sulfate, it is better to practice equalizing charges carried out regularly as a preventive measure.
Elimination of abnormal sulfation by recharging batteries with low current. Batteries that need to be treated to eliminate abnormal sulfation must first be thoroughly examined to determine that the cause of sulfation is not due to contamination of the sulfuric acid solution. The desulfation process is a relatively slow process, and therefore it must be carried out by sending a small current to the battery for a very long time (SEVERAL DAYS).
Motorists often encounter a problem such as battery sulfation. In fact, this is a completely natural process that occurs when using a battery. Because of this, the device loses capacity. However, sulfation can also be accelerated. In this case, the battery can become unusable very quickly. Therefore, it is important to know about the rules of battery care so that it lasts as long as possible.
Sulphation of battery plates - how to eliminate?
So, the main problem with lead batteries with sulfuric acid electrolyte is sulfation. While the plaque is insignificant, it can be removed at home. The crystals clogged the porous surface of the lead. They can only be extracted by breaking them down into ions and directing them to different electrodes. Used:
- exposure to reverse currents or battery restoration with pulsed charges;
- desulfation with low current for a long time;
- chemical sludge solvents;
- mechanical descaling of plates.
At home, to eliminate battery sulfation, you can use long-term exposure of the battery to a current of 2-3 A, preventing the cans from boiling. The procedure is carried out for 24 hours and further until the electrolyte density is stable for 5-6 hours. Carrying out 2-3 training cycles can return up to 80% capacity to a battery that is not completely clogged.
Ferrous sulfate precipitate dissolves well in a solution of ethylenediaminetetraacetic acid (Trilon B). The lead in the salt is replaced by sodium ion, and it becomes soluble. The solution is prepared in the ratio of 60 g of Trilon B powder + 662 ml of NH4OH 25% + 2340 ml of distilled water.
To remove sulfation, pour the solution into the battery for 60 minutes, immediately after removing the electrolyte. The reaction in the jars is violent, with heating and boiling. After draining the solution, rinse the cavities 3 times with distilled water and add fresh electrolyte. If the lead plates are not destroyed, the plates will be completely cleaned.
Light deposits can be removed using distilled water. The contents of the cans must be completely removed by pouring into an enamel container. If the contents of the jar contain coal chips, it will not recover; the plates are destroyed.
Fill the jars with electrolyte, leave the plugs open, connect the charger, set the voltage to 14 V. Make sure that the boiling in the jars is moderate, and leave for a week or two under load. The dissolved precipitate turns water into a weak electrolyte. To get rid of sulfation, repeat the procedure several times. Finish cleaning as soon as all the sediment on the battery plates has dissolved.
Single and double polarity reversal is used in cases where other cleaning methods have not helped. Changing the charge on the plates will help dissolve the precipitate by changing the direction of electron movement. But this method will destroy the battery with thin lead plates. Does not apply to modern budget models made in China.
When using special additives that dissolve sediment, you must strictly follow the instructions, carry out work in a ventilated area, and use personal protective equipment.
Why you shouldn’t let your battery reach a deep discharge state
Battery discharge is a completely natural and normal phenomenon. After all, batteries are designed to accumulate energy, release it, and then accumulate it again. And so it goes cyclically. That is, batteries are multi-chargers. There is no need to change the battery every time it loses its charge. After all, she makes up for it.
But the design of modern batteries is far from perfect. It has a number of problems and requirements:
- Recharging is not allowed, as this provokes shedding of the plates;
- It is extremely undesirable to let the battery become deeply discharged;
- It is always important to maintain the correct electrolyte density;
- the working fluid must be at a stable level;
- avoid shorting cans, etc.
How long the battery can last if a car battery is deeply discharged largely depends on the battery itself, its current condition and the efficiency of resuscitation actions.
Before you find out what to do in such a situation, it is necessary to clarify the reason for such a high danger of a deep (complete) discharge of the starter battery.
Acid batteries contain an electrolyte that has a certain density. The electrolyte is presented in the form of a mixture of sulfuric acid and distilled water.
As the battery discharges, acid gradually begins to settle on the positive lead plates in the form of salt. And the stronger the discharge, the more active and voluminous these deposits are. The density drops, significantly different from the norm.
The optimal density indicator is considered to be 1.27 g/cm³.
A deep discharge can be described as the minimum threshold for battery discharge, below which there is simply nowhere to go. If the battery is set to zero, a chemical process takes place inside, stimulating the deposition of salts on surfaces. To remove deposits, you must connect the battery to a charger as soon as possible. Or let it start charging from the car's generator.
Thus, the density is normalized, the salt crystals are destroyed, and the battery’s performance is restored.
It would seem that with a deep discharge, you can simply connect the battery to the charger, and everything will return to normal. This is a common misconception.
At zero charge, the density of the salts increases so much that upon subsequent charging they are no longer destroyed, but are firmly deposited on the surfaces of the plates.
That is, the lead plate is almost completely covered with a solid salt layer. And since the battery is charged due to the interaction of lead and electrolyte, in such a situation the battery will no longer be charged.
Such a battery is no longer capable of accumulating a charge.
With each deep discharge, the battery loses 2-3% of its capacity, which can no longer be restored.
Because of this, when the battery experiences about 10 full discharges, it is no longer possible to count on 30% capacity. With such losses, the accumulated charge is not enough to start the engine.
A discharge of up to 10.5–11 V is considered deep. It is this threshold that is considered critical when the sulfation process actively begins to occur. That is, a precipitate in the form of salt crystals begins to appear.
How to remove sulfation from a car battery, instrumentally
Battery desulfation is carried out using electrical impulses that destroy the crystal structure. In this case, the electrolyte does not drain. It is only important to make sure that the cause of the loss of capacity was the appearance of lead sulfate sediment and not the destruction of the plates or a short circuit.
Using a special charger, no additional steps are required. You need to install and connect the battery. Applying an alternating charge in a ratio of 1:10 at a set frequency will gradually clean the plates. The process is lengthy, but the result is reflected on the display with information about the restored capacity.
The scheme for removing sulfation from a battery using a conventional charger looks like this:
- Bring the electrolyte level to normal with distilled water.
- Connect the charger, set the voltage to 14 V, 1 A, charge for 8 hours.
- Measure the voltage, if it is less than 10 V, the battery will not be restored. Leave the battery to “rest” for a day.
- Connect a 14 V 2.0-2.5 A charger, keep charging for 8 hours. As a result, the voltage at the terminals should be 12.7-12.8 V. The electrolyte density should be 1.13 g/cm3
- Connect the discharge resistance, reducing the voltage at the terminals to at least 9 V.
Repeat the cycle until the electrolyte density increases to 1.27 g/cm3. Due to the gradual dissolution of the crystals that caused sulfation, the battery plates become porous. As a result, it is possible to remove additional resistance and restore the functionality of the battery.
Preventive measures
The service life of any battery is approximately 5 years. After this period, the sulfation process is inevitable, but it can be slowed down a little. To do this, it is advisable to follow simple operating rules.
- do not leave the battery for a long time without charging,
- Do not abuse the charge with high current. If the need arises, it is best to use the so-called accelerated charge mode,
- do not allow the battery to become completely discharged,
- monitor the electrolyte level,
- Pay special attention to the battery in winter.
Manual removal of sulfation from lead batteries
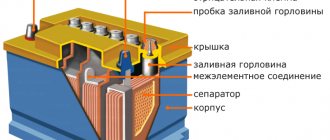
On old batteries, where the plates were collected in separate jars, mechanical removal of sediment is rarely used. How to remove sulfation manually? The battery case is disassembled, the plates are removed from the cans and cleaned manually. This is how you can tidy up a plaster-covered Ca+/Ca+ battery by first cutting off the non-removable cover with a grinder.
Manual removal of battery sulfation gives better results than using chemical additives - they remove lead not only from deposits. The active mass becomes depleted and the battery life is reduced. But during mechanical assembly there is a danger of inaccurate setting of gaps and subsequent short circuits.
Is it always possible to get rid of sulfation?
Each device has a certain operational resource, which sooner or later ends. In lead acid batteries, it most often runs out due to sulfation. This process is reversible, but sulfation cannot be completely eliminated. Part of the active mass of the electrode will certainly precipitate and will no longer take part in the generation of current, which is especially typical for cleaning with chemical reagents. Over time, the amount of reagents that generate current will become insufficient for the battery to fully perform its functions, and no amount of desulfation will help.
Battery additive against sulfation
Is it possible and how to get rid of or reduce sulfation of car battery plates using additives? There are several compounds that reduce battery sulfation, but have a negative effect on other characteristics. Soluble sulfates of active metals zinc, cadmium, and tin are used as additives to the electrolyte, but they do not reduce self-discharge and gas separation. Complex organic compounds NTP, HEDP with metal sulfates in microdoses are used as catalysts for the process of decomposition of lead sulfate crystals. Chemical additives help get rid of sulfation in a maintenance-free acid-type car battery.
Elimination methods
The process by which sulfation of battery plates is eliminated is called desulfation by experts. All manipulations associated with it are divided into several categories:
- using electric current;
- using chemicals.
Among chemical agents, the drug Trilon V is particularly effective. However, it is difficult to prepare a solution based on it, so the method could not become widespread.
It is mainly used by service specialists and experienced motorists. Methods that use electric current are much more popular today. High-amplitude pulse current sometimes helps to eliminate battery sulfation. Under its influence, the electrons of the ACT plates begin to get excited, and as a result, the lead sulfate is simply knocked out. A device for such treatment can be purchased at almost any auto supply store, but such a purchase may not be economically feasible, because in advanced situations they are not able to eliminate sulfation.
Another effective way is to repeatedly recharge the battery with low currents. For this purpose, a special charger is used, in which it is possible to regulate the power of the output current.
There are other methods, but only experienced drivers can handle them.