For such a battery, diagnostics and maintenance can be organized at home. For this purpose, you will need a special device - a hydrometer.
What is a hydrometer
A device for measuring the concentration of various solutions is called a hydrometer. The operation of the density meter is implemented on the basis of a well-known physical law.
Archimedes' principle states that if a body is immersed in a liquid, a hydrostatic or lifting force will begin to act on it. In modulus it will be equal to the weight of the liquid that the body displaced, but opposite in direction.
For practical use, the hydrometer scale is graduated to the concentration of the dissolved substance. Car enthusiasts will find a useful device for checking the density of electrolyte and antifreeze.
Types of alcohol meters
First, before choosing an alcohol meter, you need to know which type is right for you. Possible options:
- A household alcohol meter is used to check the alcohol content from zero to ninety-six degrees in liquids such as vodka, moonshine, and so on.
- A shot glass, unlike a household appliance, measures the amount of alcohol content of an alcoholic drink in small containers such as shot glasses, shot glasses, and so on.
- Laboratory - this is the most high-precision device and is used in production and for scientific work.
- Optical - used to check the strength of alcoholic drinks containing sugar and various fruit liqueurs up to 40 degrees.
- A wine meter is used to check the alcohol content of wine. The device measures not only its amount in the product, but also how much sugar it contains. This alcohol meter is not suitable for testing strong drinks, because its reading scale is from 0 to 12% alcohol and from 0 to 25% sugar content.
Read also: How to make a screwdriver work from the network
Electrolyte and its properties
The battery is an important part of the car, which is responsible for fast guaranteed starting in all weather conditions and continuous operation of all electrical modules and systems. A vehicle cannot be considered reliable if the battery is dead.
It is necessary to regularly diagnose and maintain the power source: check the level and density of the electrolyte in the battery, replenish with water, adjust the concentration, charge the battery in a timely manner.
Definition
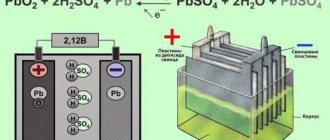
In car power supplies, a sulfuric acid solution is used as an electrolyte. It must be made with distilled water. It creates an environment for energy accumulation. The quality of the electrolyte determines the number of operating cycles of the battery, its reliability and durability.
Important! You should also monitor the amount of fluid in the battery, especially in summer. The solution should rise one centimeter above the separator plates.
Density
The more sulfuric acid is present in a solution, the higher its density. It is measured in g/cm3 or kg/m3.
The optimal electrolyte density is selected individually for each specific drive. The operating instructions indicate only recommended values.
Selecting and maintaining the correct value is very important because:
- low density leads to a decrease in device capacity and the need for frequent recharging;
- high density contributes to the rapid destruction of the drive: a large amount of acid causes the active layer of the battery plates to shed.
With the arrival of winter, this task becomes even more difficult.
Attention! To avoid unexpected troubles on the road, it is necessary to measure the density of the electrolyte in the battery once a quarter or more often. Even with minor deviations, it is necessary to charge the battery.
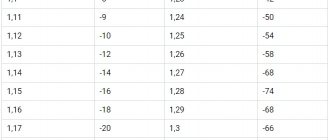
You can focus on the following density standards for different regions during the summer and winter periods:
- in warm climate zones – 1.23 g/cm3 in summer and 1.25 g/cm3 in winter;
- in cold regions – 1.27 g/cm3 in summer and 1.29 g/cm3 in winter;
- in the middle zone from 1.25 g/cm3 in summer to 1.27 g/cm3 in winter.
Important! The density of the electrolyte depends on the season and temperature of the solution.
Dependence of density on charge level
The density of the electrolyte also depends on the state of the energy source. When the battery is discharged, the concentration of sulfuric acid decreases as it is converted to lead sulfate. Accordingly, the density of the electrolyte decreases. When charging a battery, the opposite chemical process occurs.
Attention! To obtain the correct values, it is customary to measure the density of the electrolyte when the battery is fully charged. Optimal conditions for taking readings: 2-3 hours after the end of the charging process, at a temperature of 25ºC. A new certified battery at a temperature of 25ºC should show 1.28 g/cm3 for the middle band.
How to measure electrolyte density
Regular competent care can provide 10 years of reliable battery operation. For self-service, you need to know how to use a battery hydrometer and promptly check the acidity of the electrolyte.
Device design
The electrolyte hydrometer is designed as follows:
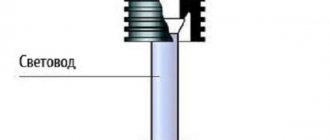
- The basis of the device is a glass tube.
- On one side of the tube there is a rubber pipette. Its long spout easily reaches the battery plates. It is made of rubber, which prevents damage to the internal parts of the power source.
- A rubber bulb is installed on the other side of the glass flask. This is a pump with which the cavity is filled with electrolyte.
- There is a pycnometer float inside the tube and a paper scale on top. The hydrometer scale is calibrated for an acid solution in kg/m3.
Security measures
Before taking hydrometer readings, you must thoroughly study the safety rules and prepare accordingly. Acid is a caustic and aggressive substance, if it gets into exposed areas of the body, it can cause severe chemical burns. Electrolyte can irreversibly damage clothes and shoes.
Be sure to wear safety glasses, rubber gloves, boots and an apron!
Attention! Smoking is strictly prohibited during work. The acid contains hydrogen. It's explosive!
If affected by sulfuric acid, it must be washed off with running water as quickly as possible and very thoroughly for 10–15 minutes. If possible, it will be effective to neutralize the acid with an alkaline solution, such as soap and water or a 10% soda solution, before rinsing.
Instructions: how to use a hydrometer correctly
You can take readings in a car, but it is better to install the battery in a room with good ventilation on a stable horizontal surface.
You must work with the battery carefully: protect it from shock, do not turn it upside down, avoid short circuits.
To check the density of the electrolyte in the battery, you must perform the following steps:
- First you need to assemble the device.
- Remove the caps from all battery cans.
- If there is a low level of liquid in any jar, it must be refilled with distilled water before measuring.
- Lower the pipette and draw enough liquid into the flask so that the float floats freely vertically without touching the walls of the vessel. The lower the acidity, the lower it will go.
- Read off the scale at the liquid level. At this time, the hydrometer must be kept motionless and vertical.
Attention! After each measurement, the device must be thoroughly washed to remove any remaining acid and antifreeze. This will ensure its long-term and reliable operation.
Operating principle of a hydrometer
Checking the density of the battery with a hydrometer is done by immersing a float-pycnometer in the substance being measured and determining the ratio of the mass of the liquid to its volume. It is necessary to record readings after equilibrium has been achieved.
Interpretation of the result
If the electrolyte density readings in one of the battery cans are lower than the others, this means that there is a short circuit in it. Accordingly, the battery is not working properly. It cannot be restored.
An equal density value in all jars (±0.01 g/cm3) characterizes the degree of its charge and the need for certain actions.
Low values of the entire battery may be due to the following problems:
- the drive is very discharged;
- the battery is defective;
- The battery is outdated and needs to be replaced;
- The power supply is very worn out because the engine is constantly running on it.
DIY alcohol meter
However, if there is no opportunity or desire to buy such a device, then you can make it yourself. And we can say with confidence that homemade alcohol meters are not much inferior to factory instruments.
From a ballpoint pen
To make such a device you will need:
- a ballpoint pen case (the simplest one) no more than 14 centimeters in length;
- a plastic container for nasal drops and small shot (you can also use finely chopped steel wire or finely chopped lead for weight).
The following is done:
- The lower part of the handle is cut off by half a centimeter.
- To make it easier for the pen case to fit into the neck of the drop bottle, you need to hold it in hot water.
- The pen case is carefully screwed into the neck of the bottle.
- The workpiece is lowered into a container with warm water and a weighting agent is loaded through the upper hole until it is immersed in water one and a half to two centimeters from the neck. A mark is made on the case (it will be 0).
- Carefully bury the glue inside to fix the weighting material and screw the cap tightly.
- The alcohol meter is immersed in a container with 40-degree vodka. Mark 40.
- Use a ruler to measure the distance from 0 to 40 and divide by 40. This reading will correspond to one degree.
- Using the data obtained, a scale is formed. The alcohol meter is ready.
Alcohol meter from a fishing float
To make an alcohol meter from a fishing float you need:
- Take a float with a long pole. If necessary, attach a weight to the lower part to lower the float to the desired level.
- Place it in water at room temperature, put a mark that will be equal to zero.
- Pour 40 degrees vodka into a deep container, lower the float there and also put a mark. And, using the principle of an alcohol meter, form a scale from a ballpoint pen.
Test tube alcohol meter
To make such a device you will need:
- test tube;
- weighting agent;
- test tube stopper and wooden rod 12–15 centimeters.
The next steps are:
- It is necessary to load the weighting agent into the test tube so that it is completely immersed in the liquid, but does not sink to the bottom, but floats near the surface.
- Secure the weighting agent with glue.
- Close the test tube with a stopper and insert a wooden rod into the stopper.
- According to the manufacturing principle of the above instruments, apply a scale to the rod.
In conclusion, we can say that homemade alcohol meters are practically in no way inferior to domestically produced factory instruments. Moreover, they work properly, unlike their Chinese counterparts, which are consistently inaccurate in their readings. It’s up to you to decide which device to use.
Hydrometer models for electrolyte
Today, a wide variety of hydrometer models are produced. For personal use, it makes sense to purchase a simple, inexpensive device.
The most popular budget models among motorists, costing no more than 600 rubles:
- Neat and affordable hydrometer for electrolyte Sparta 549125.
- The Chinese inexpensive device Skybear 623000 received a large number of positive reviews.
- The domestic instrument Orion AR-02 has a noticeable drawback: the float sticks to the tube, this fact creates inconvenience.
There is a digital version - refractometers. They are expensive, 800 rubles and more. They are mainly used by professionals. Digital instruments can be adjusted using distilled water and can determine parameters very accurately.
The density of the electrolyte affects the reliability and service life of the battery. Knowing how a hydrometer works, you can independently, regularly and correctly monitor the acidity level and service the battery, you can extend the life of the device by several years, and always feel confident and reliable behind the wheel.
You may also be interested
.
Batteries 0