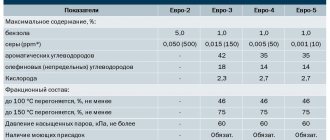
Octane number
The name of the gasoline brand consists of an alphanumeric designation. The letters A or AI indicate the method for determining the octane number:
- motor (A)
- research (AI)
and the number determines the octane number (92, 95, etc.).
The octane number indicates a property such as gasoline’s resistance to detonation. This figure is relative. Iso-octane is taken as a standard, the detonation resistance of which is very high and is taken to be equal to 100. The octane number scale was proposed at the beginning of the last century. It was determined by the content of isooctane in a mixture with normal heptane (its detonation resistance is very low and is assumed to be zero). Accordingly, AI-92 gasoline is equivalent in its resistance to detonation to a 92% mixture of isooctane and heptane, AI-95 - 95%, and so on. The octane number can be more than 100 if the anti-knock properties of the fuel are even higher than those of pure isooctane.
This value is very important, since detonation leads to rapid destruction of the cylinder-piston group. This is explained by the speed of propagation of the flame front - up to 2.5 km/s, whereas under normal conditions the flame spreads at a speed of no more than 60 m/s
To increase anti-knock properties, you can either add additives containing lead compounds (tetraethyl lead) or change the fractional composition upon receipt. The first method can be easily obtained from AI-92 gasoline, AI-95, or 98, but has now been abandoned. Because, although such additives significantly increase the performance properties of fuel and have a low cost, they are also very toxic and have a much more detrimental effect on the environment than pure gasoline, and also destroy the car’s catalytic converter (the combustion temperature of leaded gasoline is higher than that of unleaded gasoline, As a result, the ceramic elements of the neutralizer simply sinter, and the device fails).
Other compounds that are less toxic, such as ethyl alcohol or acetone, can also be used as additives. For example, if you add 100 ml of alcohol to a liter of AI-92 gasoline, the octane number will increase to 95. However, the use of such additives is not economically profitable.
HOW CAN WE PREVENT FUEL PIPE FREEZING?
To avoid all the hassle of thawing a frozen fuel line, prevention is key.
Here are some tips on how to keep your fuel line from freezing while storing your car in your garage:
KEEP THE TANK FULL
This reduces moisture in the system.
After all, water vapor is the main culprit in freezing fuel lines.
Condensation produces water and we all know that water freezes at cold temperatures.
- ADD ANTIFREEZE TREATMENT.
Antifreeze is both a solution and a preventative when it comes to frozen fuel lines. Just make sure you follow the product instructions.
About temperature and freezing
What is better to fill the tank with 92 or 95 gasoline Let's figure out which gasoline is better
The oil itself becomes thick at -25 - 30 degrees Celsius, but gasoline is its own, if you want a volatile compound, it has a much lower freezing level.
For the middle zone, where the frost is about -20 - 35 degrees, you don’t have to worry about anything; freezing gasoline in the tank in the cold is simply physically impossible here.
To be honest, in the Arctic, there is a special gasoline, it is called “Arctic”. Its threshold is even lower, so with the help of a special formula and additives it remains liquid up to -150 degrees Celsius, which is more than enough, yet there are practically NO such temperatures on earth!
But freezing of the fuel is not the worst thing - it needs to leave behind the possibility of ignition, otherwise it will be of little use, the so-called viscosity! Thus, Russian GOSTs (GOST R 51105-97 and GOST R 51866-2002 - “as amended”) characterize not only the sulfur content in the fuel, but also the minimum temperature at which a flash should occur in the engine cylinders, now it is 62 degrees. That is, at this temperature, gasoline of popular brands should ignite and not thicken.
Preventing fuel system freezing
At what temperature does antifreeze boil?
Knowing at what degrees gasoline freezes, a person will be able to effectively prevent freezing. For prevention you will need to do the following:
- refuel only at gas stations with a positive reputation;
- when refueling from a canister, use funnels with a mesh to filter the fuel;
- If the car has a diesel engine, then in winter you need to use a frost-resistant type of diesel fuel.
Not all motorists know that there is a summer diesel engine (can be used at temperatures down to -5 degrees) and a winter diesel engine (can be used at temperatures down to -35 degrees).
To prevent impurities and water from getting into the gas tank, sometimes it is not enough to refuel at proven gas stations. Even high-quality gasoline can contain a small amount of unnecessary elements. To combat freezing of the fuel system, you can use pure alcohol. It is poured into the gas tank in the proportions of 1 liter of alcohol to 10 liters of gasoline. The alcohol will help bind excess water molecules. However, it will not harm the car. Prevention with alcohol is carried out 1-2 times a year.
You can use a transparent container to evaluate fuel when visiting a new gas station. Before refueling your car, you will need to pour gasoline into a transparent plastic container. If no impurities are found during inspection, you can pour fuel into the tank to refuel the car without fear.
It is important to know the temperature at which gasoline freezes. This will help you quickly determine the cause of the problem, which may be related to the fuel pump and the rest of the fuel supply system.
Using special additives, it is possible to significantly reduce the temperature at which liquids freeze.
In the Arctic regions, special gasoline is used that does not freeze even at -120 degrees.
No place on the planet has ever recorded such a low temperature. In global human habitats, ordinary fuel also rarely changes its state. It does not lose its characteristics over a long period of time.
Fuel - wide fractional composition
At what temperature should tires be changed?
Fuels of a wide fractional composition have the significant disadvantage that they have increased volatility and high saturated vapor pressure. As a result, when working on fuels of a wide fraction composition, some difficulties arise associated with their evaporation and boiling at high altitudes; however, when flying at altitudes up to 10 - 12 km, the use of fuels of a wide fraction composition, having a vapor pressure no higher than 100 - 150 mm Hg. Art., quite acceptable.
Fuels of a wide fractional composition and type of kerosene, as a rule, are products obtained by direct distillation of oil.
T-2 fuel of a wide fraction composition, containing mercaptans, has a corrosive effect on copper to a greater extent than TS-1 fuel.
The application is for JP-3 fuel of a wide fraction composition with a vapor pressure of 267 - 374 mm Hg. Art., but increased vapor pressure makes it difficult to use this fuel at high altitudes at low pressure. JP-4 and JP-5 fuels, which have a vapor pressure of 107 - 160 mm Hg, do not have this disadvantage. Art. They are obtained by direct race of thermal and catalytic cracking products, hydrocracking. The temperature at which JP-5 fuel begins to crystallize is increased to -40 C and its fractional composition is heavier compared to other fuels.
The soot-forming ability of fuels of a wide fractional composition (fuels B, JP-4) is characterized by the smoke and volatility index, numerically equal to the sum of the height of the non-smoking flame of the fuel (in mm) and the product of the coefficient 0 42 by the number of fuel fractions (in vol.
Currently, fuels of a wide fraction composition are obtained from some gas condensates and are used in remote northern and northeastern regions of the country, where delivery of standard diesel fuel is difficult.
The first is typical only for fuel of a wide fraction composition; When burning fuel that is distilled within very narrow temperature limits, or even more so a single-component fuel, the same fuel components enter all cylinders, even if the fuel does not evaporate completely. In the second case, only with complete evaporation of the fuel and good mixing of its vapors with air can the same, corresponding to the specified, composition of the working mixture be ensured for different cylinders. If the mixing of fuel vapors with air is not intense enough, and even more so if the volatility of the fuel is insufficient, then even when operating on a single-component fuel, the composition of the working mixture in individual cylinders will be different. The greatest unevenness of the fuel components and the composition of the working mixture occurs when there is a fuel film on the walls of the intake pipe.
When assessing the carbon-forming ability of fuels of a wide fraction composition, their volatility is important. Therefore, the soot-forming ability of these fuels is assessed by the value of the soot formation index, which relates the smoke point and the volatility characteristics of the fuel
Tests have shown that there is a relationship between the carbon formation index and the amount of carbon deposits in the engine, as can be seen from the figures below.
They are produced in two grades: JP-4 - fuel of a wide fraction composition and JP-5 - kerosene with a high flash point.
Among jet fuels, fuels of a wide fraction composition such as T-2 and JP-4, which contain gasoline fractions and have a saturated vapor pressure in the range of 80 - 160 mm, are characterized by better volatility. Low volatility is characteristic of fuels such as kerosene TS-1, T-1, T-5, T-6, T-7, JP-1, JP-5 and JP-6, etc., which have an initial boiling point of 130 - 195 and saturated vapor pressure, no more than 40 mm.
Jet fuels such as aviation kerosene and fuels of a wide fraction composition always contain some amount of sulfur, nitrogen and oxygen compounds.
Calculation methods are unsuitable for determining the CN of fuels of a wide fraction composition containing gasoline fractions, as well as fuels with additives that increase the CN.
For aircraft with turboprop engines, fuels of a wide fraction composition can be used. JP-4 fuel is recommended for supersonic aviation at flight speeds up to 1800 km/h, JP-5 fuel, as a heavier fuel, is recommended for speeds up to 3600 km/h, while some of the fuel can be supplied to the engine in evaporated form due to significant heating aircraft and its tanks during aerodynamic braking.
For aircraft with turboprop engines, fuels of a wide fraction composition can be used.
| Dependence of median drop diameter on ratio. nozzle radius / siu a / 2.| Distribution coefficient for pressure injectors. |
Why gasoline as a fuel?
Why not! This is a highly energetically valuable product, you know - that a liter of this fuel can produce 10,500 - 11,000 kilocalories of energy, just think about it! For example, this is enough to lift a weight of 4000 tons to a meter height (taking into account the correct supply of energy). Therefore, a long time ago it was decided to build internal combustion engines using one of the types of hydrocarbon fuels. To be fair, all three types are now used: kerosene, gasoline and diesel .
This is why it is so difficult to replace internal combustion engines with electric ones. Despite the high efficiency of the electric motor, it requires serious energy injections from batteries, and in our time, to my great regret, they are NOT PERFECT.
Evaporation is the fuel
Plastic canisters of gasoline in the trunk
The heat of evaporation of fuel has a significant effect on the weight filling of the engine cylinders with fresh mixture. Related to this is the use of fuels having a high heat of vaporization as fuels for racing cars. The heat of vaporization is measured in calorimeters.
The completeness of fuel evaporation during the formation of a combustible mixture depends on the chemical composition of the fuel, as well as on design and operational factors.
The rate of fuel evaporation depends on the quality of atomization, turbulence of gas flow, temperature and volatility of the fuel. Depending on these factors, evaporation and combustion of fuel in the engine may be complete or incomplete.
| Intake manifold with filter. |
For better fuel evaporation, additional heating of the combustible mixture is organized with exhaust gases or water coming from the cooling system and passing between the double walls of the intake pipe.
For better evaporation of fuel, heating of the combustible mixture is provided in the intake pipe. For this purpose, in the middle part there is a heating chamber with double walls, between which exhaust gases circulate, entering through a special window from the exhaust pipeline.
For reliable evaporation of fuel in the working mixture, heating of the incoming air is required, and the required degree of heating depends on: 1) the type of fuel, 2) the composition of the working mixture and 3) the state of the outside air.
When fuel evaporates in a confined space, vapor condensation simultaneously occurs.
When fuel evaporates, moist gray vapors are formed, gradually mixing with air moving towards the cylinders.
When fuel evaporates in a confined space, vapor condensation simultaneously occurs.
When fuel evaporates, its molecules fly out of the liquid into the surrounding air. Some of the evaporated molecules may hit the surface of the liquid again and be absorbed by it. The degree of fuel evaporation is determined by the difference between the number of molecules emitted from the liquid and reabsorbed by it. The intensity or rate of evaporation depends on the initial concentration of molecules of a given fuel in the air and on the rate of their diffusion. If the gas space above the liquid is not limited, then evaporation occurs at maximum speed. In this case, free evaporation takes place. In a closed volume, at the initial moment the evaporation rate is equal to the rate of free evaporation, but as the air is saturated with fuel molecules, the number of molecules returning back to the liquid phase increases, and the evaporation process slows down. At a certain concentration of fuel molecules in the air, the number of molecules leaving the liquid and returning to it is equalized, and a state of dynamic equilibrium occurs.
When fuel evaporates, its molecules fly out of the liquid and either leave it completely, diffusing into the environment (air), or, hitting the surface of the liquid, are again absorbed by it; in this case, only a small part of the molecules is absorbed, characterized by the coefficient of accommodation of vapor molecules by the liquid.
When fuel evaporates, its molecules fly out of the liquid into the surrounding air. Some of the evaporated molecules may hit the surface of the liquid again and be absorbed by it. The degree of fuel evaporation is determined by the difference between the number of molecules emitted from the liquid and reabsorbed by it.
| Dependence of the specific latent heat of evaporation of normal paraffin hydrocarbons on their temperature. |
Of course, fuel evaporation can occur at a lower or higher temperature than this, but the overall effect, which we can only judge, is the same as if the fuel evaporation occurred precisely at this temperature. It can be assumed that this temperature will be close to the equilibrium temperature of the drop, when the rate of cooling of the drop due to evaporation will be equal to the rate of its heating due to heat transfer from the air.
Fractional composition - fuel
| Disc with cups. |
The influence of the fractional composition of the fuel on its varnish formation is clearly visible in the example of direct petroleum distillation products: gasoline produces the least amount of varnish, kerosene is more prone to varnish formation, and diesel fuels form the largest (in mg per 10 ml of fuel) amount of varnish.
| Evaporation losses of jet fuels depending on their temperature. |
The fractional composition of the fuel is also related to its calorific value.
| The influence of fuel vapor pressure on pump performance. |
The fractional composition of the fuel is also related to its calorific value. It can be referred to as a unit of volume and a unit of weight. As already indicated, jet engines, while providing high aircraft speed at significant altitudes, are characterized by high fuel consumption, and the range of aircraft largely depends on the required fuel supply. Since the volume of fuel tanks in jet aircraft is limited, the volumetric calorific value of the fuel is primarily important.
The fractional composition of the fuel is closely related to the flash point, at which vapors of a petroleum product with air form a flammable mixture that flares up when a fire is applied. The fuel is heated and a pilot light is periodically brought to its surface. The flash point is determined by the moment a rapidly disappearing flame appears on the surface.
| Fractional composition of diesel fuel. |
The fractional composition of the fuel is closely related to the flash point, at which vapors of a petroleum product with air form a flammable mixture that flares up when a fire is applied. The flash point is determined (GOST 6356 - 75) in a closed type device. The fuel is heated and a pilot light is periodically brought to its surface.
The fractional composition of fuels is especially important for high-speed diesel engines, since in these engines an extremely short time is allocated for the complete combustion cycle of the fuel-air mixture. As a rule, the higher the diesel engine speed, the lighter the fuel it requires. The fuel boil-off limit is limited by normal combustion conditions; Excessively large amounts of light or heavy fractions in diesel fuel negatively affect the combustion process. If the fuel contains too many light fractions, the pressure in the engine cylinder increases greatly; this causes sharp knocking noises in the cylinder, and the diesel engine becomes rough. An increase in the content of heavy fractions leads to incomplete combustion of fuel (due to the short duration of the combustion cycle), and the engine becomes polluted with products of incomplete combustion.
As the fractional composition of the fuel becomes heavier and the content of aromatic hydrocarbons in it increases, the amount of sediment increases. This is due to incomplete evaporation and combustion of fuel.
The degree of influence of the fuel fractional composition on engine operation largely depends on its operating mode and ambient air temperature. When operating in winter without heating the working mixture with the throttle closed or in frequently changing mode, the influence of the fractional composition of the fuel is especially pronounced.
| The influence of fuel vapor pressure on pump performance. |
With a lighter fractional composition of the fuel, or more precisely with an increase in its vapor pressure, the performance of the pumps deteriorates. As can be seen from the curves in Fig. 153, pump performance on fuel with a vapor pressure of 360 mm Hg. Art. lower than with fuel with virtually zero vapor pressure; The decrease in productivity with decreasing head pressure occurs more sharply. The size and weight of the pump are proportional to the ratio of steam to liquid fuel. Therefore, to pump fuel with a vapor pressure of 360 mm Hg. Art., pumps are required that are 2 - 4 times larger in size than for pumping kerosene.
Excessive lightening of the fractional composition of the fuel has no less harmful effect on the performance of a diesel engine than excessive weighting.
What to do if the fuel in the tank is frozen
Normally, moisture is present in any fuel tank, but water does not always have a negative effect on engine performance. As has already been found out, gasoline freezes at a temperature of minus 60 C. If it is discovered that the fuel in the tank has become thick or completely frozen, then it is necessary to take measures to eliminate what happened. Start warming up the car in every possible way. Use an electric hair dryer, drive the car to an underground parking lot or a heated garage. After defrosting, drain the low-quality fuel and fill it with new one. If you use diesel fuel, it has a greater chance of freezing. If signs of icing appear, pour half a spoonful of engine oil into the tank of a diesel car. The moisture will evaporate after the emulsion burns.
Evaporation is the fuel
Evaporation of fuel in diesel engines begins immediately after its injection into the combustion chamber and continues until the last portions of fuel are burned. It takes 10 times less time to prepare the combustible mixture in a diesel engine than in a carburetor engine, and at the same time, a diesel engine manages to use heavier fuels with worse volatility. This is explained by the fact that in diesel engines, well-atomized fuel is injected into air heated by compression to 500 - 600 C. Such conditions ensure intense heating and evaporation of fuel droplets.
Evaporation of fuel in the air flow is also directly dependent on the rate of vapor diffusion. Different types of fuel have different coefficients.
Evaporation of fuel is carried out at a regulated (high) temperature in a stream of gas - air or steam. In this case, hydrocarbons are not strictly separated from the resins according to their boiling temperatures. The gas flow contributes to the entrainment of some of the resinous compounds with hydrocarbon vapors, and the action of high temperature, especially when blowing with air, causes the oxidation of hydrocarbons and the new formation of resinous substances during analysis.
Evaporation of fuel occurs mainly when the engine is not running.
Evaporation of fuel in diesel engines begins immediately after injection into the combustion chamber and continues until the last portions of fuel are burned. It takes 10 times less time to prepare the combustible mixture in a diesel engine than in a carburetor engine, and at the same time, a diesel engine manages to use heavier fuels with worse volatility. This is explained by the fact that in diesel engines, well-atomized fuel is injected into air heated by compression to 500 - 600 C. Such conditions ensure intense heating and evaporation of fuel droplets.
Evaporation of fuel begins immediately after it leaves the injector. At this moment, the evaporation rate is slightly affected by the temperature of the air entering the root of the torch.
Evaporation of fuel in the internal combustion engine occurs with simultaneous heat exchange.
Evaporation of fuel into the washing gas flow occurs from the surface of the film as a result of its heating from the wall of the combustion chamber. In order for evaporation to occur quickly enough, but without thermal decomposition of the fuel, the wall temperature must be maintained within 200 - 400 C.
| Influence of air speed |
Evaporation of fuel begins immediately after it leaves the injector.
After the fuel evaporates, resins remain, which are determined by weighing on an analytical balance. The resin content in direct race jet fuels of the T-1 type should not exceed 3 mg per 100 ml of fuel.
Heat is expended on fuel evaporation, as a result of which the temperature of the fuel and air decreases during evaporation. The degree of cooling is proportional to the amount of evaporated fuel, its latent heat of evaporation, inversely proportional to the amount of air per unit weight of the fuel and the heat capacity of the fuel.
| Direct fuel injection system. |
With direct injection, less time is allocated for fuel evaporation. Factors that accelerate evaporation are increased vortex movement of air, high temperature inside the cylinder and low pressure during the suction stroke. During the compression stroke, the vortex movements die out, but the temperature at the time of ignition rises and can reach 400 C.
| Dependence of saturated vapor pressure of gasoline on temperature.| Total number of revolutions. |
Gasoline never freezes
There is an opinion that, unlike diesel fuel, gasoline does not freeze and does not mind any frost. However, this is not quite true. In the fall, water condensation accumulates in the tank, which sinks to the bottom of the gas tank and accumulates in the recess in front of the gas pump. It is then sucked into the fuel supply system in the form of a mixture and evaporates in the combustion chambers. After turning off the power unit, some of the fuel remains in the lines, in the filter and in the pump. During severe frosts, the water stratifies, turns into grains of frost inside the liquid and crystallizes, blocking the passage for gasoline. As a result, the car stalls.
To prevent water from accumulating in the gas tank, you can use special chemicals that are added to the fuel. They dissolve water in themselves and prevent it from freezing. You can also use food grade ethyl alcohol for these purposes.
Evaporation - gasoline
| Diagram of a simple carburetor. |
Evaporation of gasoline begins from the moment it leaves the carburetor channels into the air flow in the diffuser. Under the influence of the kinetic energy of moving air, the flowing stream of gasoline is split into separate mixtures. Small drops have time to evaporate in the carburetor mixing chamber. Larger droplets are carried away by the air flow and evaporate as the mixture moves along the intake tract and in the engine cylinders. The largest drops of fuel settle on the walls of the mixing chamber and inlet pipe, forming a liquid fuel film. The steam-air flow carries the film along the walls of the intake pipe towards the combustion chambers.
| Fuel volatility and cooling of metal carburetor parts. |
The evaporation of gasoline in the engine intake system is accompanied by a decrease in the temperature of the fuel-air mixture due to the fact that the heat necessary for the evaporation of gasoline (heat of evaporation) is taken away from the air in which evaporation occurs and from the metal parts of the intake system. It was noted, for example, that at an ambient temperature of 7 5 C, the temperature of the throttle valve two minutes after starting the engine drops to - 14 C.
Evaporation of gasoline begins from the moment it leaves the atomizer and continues in an air stream moving at high speed. In this case, part of the gasoline evaporates in the intake manifold, and part - in the engine cylinder.
| Fuel volatility etc. |
The evaporation of gasoline in the engine intake system is accompanied by a decrease in the temperature of the fuel-air mixture due to the fact that the heat necessary for the evaporation of gasoline (heat of evaporation) is taken away from the air in which evaporation occurs, and. It was noted, for example, that at an ambient temperature of 7 5 C, the temperature of the throttle valve 2 minutes after starting the engine drops to - 14 C.
Evaporation of gasoline occurs during all operations (filling, storage, refueling), the amount of losses depends on the organization of work, technical equipment and condition of the equipment.
Gasoline evaporation is closely related to vapor pressure. The lower the vapor pressure, the slower the gasoline evaporates, and vice versa. At the same time, the gasoline standard limits the most permissible vapor pressure, which should not exceed 500 mm Hg.
| Typical characteristics of gasoline evaporation from oil during continuous engine operation for 1 hour on a stand. |
The evaporation of gasoline from the oil system of an aircraft in flight with oil tanks having circulation wells occurs even faster.
Gasoline evaporation is a factor that must be taken into account when organizing the transportation process. In addition to the fact that saturation of the tank or reservoir space with gasoline vapors is dangerous in terms of fire and explosions, the evaporation of gasoline changes its qualitative composition. In addition, the evaporation of gasoline causes losses during transportation.
The higher the ambient temperature, the more intense the evaporation of gasoline occurs. Therefore, when storing and transporting gasoline, they resort to a number of measures that reduce the degree of evaporation when the ambient temperature rises. To reduce evaporation, gasoline tanks and gasoline storage tanks are painted a light color.
The evaporation of gasoline in the intake pipe is accompanied by the division of gasoline into fractions. During the intake process, mainly low-boiling fractions evaporate. They, forming a steam-air mixture, enter the cylinder. High-boiling fractions settle on the wall of the intake pipe in the form of a liquid film, which, gradually evaporating, moves along the intake tract. When using high-octane gasoline, as a result of this mixture formation process during intake (especially in unsteady conditions), low-boiling fractions with a relatively lower octane number first enter the cylinder. This may lead to detonation. The greatest influence on the distribution of gasoline, which has different detonation resistance, among the cylinders is caused by the ethylation of gasoline, which is associated with the uneven distribution of tetraethylene when individual fractions boil off.
The evaporation of gasoline in the engine intake system is accompanied by a decrease in the temperature of the fuel-air mixture due to the fact that the heat necessary for the evaporation of gasoline (heat of evaporation) is taken away from the air in which evaporation occurs and from the metal parts of the intake system. It was noted, for example, that at an ambient temperature of 7 5 C, the temperature of the throttle valve 2 minutes after starting the engine drops to -14 C.
Boiling point, combustion temperature of gasoline
Any person who decides to find information about the boiling, combustion or ignition temperature of fuel will find an interesting thing: even in fairly well-known sources there is a difference between the indicated indicators of the same parameter. Why does this happen and what are the real indicators?
Gasoline boiling point
The boiling point of gasoline is an interesting value. Today, few young motorists know that once upon a time, at high air temperatures, fuel boiling in the fuel line or carburetor could block the vehicle. This phenomenon contributed to the formation of failures in the system.
The light fractions were strongly heated and separated from the heavier ones in the form of bubbles of flammable gas. The car cooled down, the gases turned into liquid - and it was possible to continue moving. Today, gasoline used at gas stations boils at approximately +80 degrees.
Fuel flash point
The flash point of a fuel is the thermal threshold at which freely separating, lighter fractions of the fuel begin to burn from an open flame source when this source is located above the test sample.
In practice, it has been shown that the flash point is determined by the method of heating in an open crucible. The fuel to be tested is poured into a small open container. Then it is heated slowly without involving an open flame.
At the same time, the temperature is monitored in real time. Each time the fuel temperature increases by 1 degree, a flame source is used at a low altitude above its surface. At this moment, when fire occurs, the flash point is determined.
In other words, the flash point determines the threshold at which the concentration of easily evaporating fuel in the air reaches a level sufficient to ignite under the influence of an open fire source.
Gasoline combustion temperature
This indicator reveals the maximum temperature created by burning gasoline. And here, too, there is no unambiguous information that answers this question with one number. Surprisingly, it is for the combustion temperature that the key role is played by the conditions of the process, and not the composition of gasoline.
If you look at the calorific value of different gasolines, there are no differences between AI 92 and AI 100. In fact, the octane number only reveals the resistance of gasoline to the occurrence of combustion processes.
And the quality of the essence itself and its combustion temperature are not affected. By the way, often simple gasolines AI 76 and AI 80, which have gone out of use, are cleaner and safer for humans than AI 98, modified with a large number of additives.
In the engine, the combustion temperature of the fuel ranges from 900 to 1100 degrees. This is if the ratio of air and fuel is equal to the stoichiometric ratio. The actual combustion temperature can either decrease or increase under specific conditions.
The combustion temperature is largely affected by the compression level. The higher it is, the hotter it is in the cylinders. With an open flame, fuel burns at low temperatures, approximately 800-900 degrees.
Volatility - Fuel
| Installation diagram for determining the fractional composition of fuel. |
Fuel volatility is one of the main operational characteristics, as it affects the processes of mixture formation and combustion, fuel loss during high-altitude flights, and the possibility of vapor locks forming in fuel lines. The volatility of a liquid is its ability to transform into a gaseous state. It is judged mainly by two indicators: fractional composition and saturated vapor pressure.
In this case, the volatility of the fuel deteriorates. When using fuel of a heavier fractional composition in the engine (instead of the usual one), mixture formation worsens.
Fuel volatility is also of great importance in the problem of fuel consumption and obtaining maximum engine power.
The volatility of the fuel is determined by the fractional composition. Unlike gasoline, the fractional composition of diesel fuels is regulated only by the boiling points of 50 and 96% of the fuel. This is explained by the fact that no clear connection has been established between the boiling point of 10% diesel fuel and the operation of diesel engines. When the fuel is lighter, the starting of diesel engines worsens, since light fractions have worse self-ignition compared to heavy fractions. Therefore, the starting properties of diesel fuels for cars are to some extent determined by the boiling point of 50% of the fuel. The boiling point of 96% of the fuel regulates the content of the heaviest fractions in the fuel, an increase in which impairs mixture formation, reduces efficiency, and increases carbon formation and smoke in exhaust gases.
The volatility of fuels largely depends on the saturated vapor pressure and, consequently, on the fractional composition of the fuel.
Fuel volatility mainly determines the efficiency of mixture formation processes in the engine and fuel loss during production, transportation, storage and use.
| The flammability limits of certain flammable substances in air at 20 C. |
The volatility of fuels can be regulated by fractional and component composition, mainly during the production of fuels.
The volatility of the fuel is determined by the fractional composition. Unlike gasoline, the fractional composition of diesel fuels is regulated only by the boiling points of 50 and 96% of the fuel. This is explained by the fact that no clear connection has been established between the boiling point of 10% diesel fuel and the operation of diesel engines. When the fuel is lighter, the starting of diesel engines worsens, since light fractions have worse self-ignition compared to heavy fractions. Therefore, the starting properties of diesel fuels for cars are to some extent determined by the boiling point of 50% of the fuel. The boiling point of 96% of the fuel regulates the content of the heaviest fractions in the fuel, an increase in which impairs mixture formation, reduces efficiency, and increases carbon formation and smoke in exhaust gases.
| Fractional distillation curves for various fuels. |
The volatility of the fuel, depending on its fractional composition, vapor pressure, surface tension and heat of vaporization, is one of the main characteristics of the fuel. It is determined in a special device by heating the fuel and sequentially selecting fractions that boil away in certain temperature ranges.
Fuel volatility should be taken into account for another reason.
The volatility of fuels in diesel engines is of less operational importance than the volatility of gasoline in carburetor engines. This is due, first of all, to the fact that in a diesel engine, mixture formation occurs at a very high temperature at the end of the air compression stroke. 0 6 - 2 0 ms are allotted for fuel evaporation in a high-speed diesel engine. In order for the fuel to evaporate during this time, its droplet size must be in the range of 10 - 20 microns; As the droplet diameter decreases, the rate of their heating increases. The completeness of fuel evaporation in the engine depends on temperature, vortex movement of air in the combustion chamber, cutting quality and fuel volatility.
The volatility of fuel for marine gas turbine plants is as important as for other internal combustion engines. The quality of mixture formation, the completeness of fuel combustion, as well as the shape of the temperature field in the combustion chamber and related phenomena largely depend on it.
Reviews and recommendations from car owners.
Ivan: From personal observation, I will say that the critical temperature for summer diesel fuel is minus 10 °C, and at minus 10-15 °C problems begin with the fuel filter - it becomes clogged with thick paraffin flakes. In the 90s, foreign cars running on diesel fuel were already equipped with a heated fuel filter. Nowadays, diesel fuel is often bad and you can’t get enough anti-gel for it. Therefore, I chose one gas station, where they more or less don’t fill up, and that’s the only place I refuel.
Vladimir Evgenievich: I had such a problem once and it was only minus two. No fuel was supplied. I had to unscrew the tubes with the mesh from the tank. I immediately saw some kind of lump of matter with ice pieces on the mesh. Maybe even earlier liquid got into the tank along with this rag. Now I don’t lose my vigilance, I often look into the filter.
Egor: I consider diesel fuel a waste product from oil refining. If it is clean, without any impurities, then at our usual winter temperatures it cannot freeze. Gasoline will not freeze even at minus 40.
But if any obscene rubbish is added to diesel, for example paraffin or water (we often do this at gas stations), then minus 20 will be enough to freeze. Then both the engine and the car owner will have problems.
Sergey: Different types of fuel have their own temperature regimes. So summer diesel fuel is designed for operation within - 5 to - 7 degrees.
Winter - it will freeze already at -30 - -35 degrees.
Special, Arctic - only at - 50 degrees.
All car owners should know this so that they don’t have any difficulties or troubles in cold weather.
Didn't find the information you are looking for? on our forum.